Many of these products contain active moieties that. FDA Approves Trodelvy Sacituzumab govitecan-hziy for Triple Negative Breast Cancer.
 Cancer Drug Approval Friends Of Cancer Research
Cancer Drug Approval Friends Of Cancer Research
The FDA granted approval of Tukysa to the company Seattle Genetics Inc.

New cancer drug approved by fda. The US Food and Drug Administration FDA has granted accelerated approval to a new cancer drug called selinexor. Approvals of New Drug Applications NDAs Biologics License Applications BLAs and Abbreviated New Drug Applications ANDAs and supplements to those applications. To characterize RR end points used by the US Food and Drug Administration FDA for cancer drug approval.
Food and Drug Administration. The drug names link to NCIs information summaries about these drugs. The agency was besieged by calls from cancer patients and libertarian groups to.
HealthDayA new drug to treat the most common form of skin cancer has been approved by the US. The drug Nerlynx neratinib was approved for extended adjuvant treatment of early-stage HER2-positive breast cancer. The US Food and Drug Administration FDA has expanded the approval of the immunotherapy drug pembrolizumab Keytruda to include any cancer with a high tumor mutational burden TMB-H.
Food and Drug Administration approved pembrolizumab Keytruda Merck Sharp Dohme Corp in combination with platinum and fluoropyrimidine-based chemotherapy for patients with metastatic or. Beijing-based BeiGenes Brukinsa treats blood cancer New drug will compete with therapies from AbbVie AstraZeneca The US. FDA Approves Trodelvy Sacituzumab govitecan-hziy for Triple Negative Breast Cancer.
A new drug to reduce the risk of breast cancer recurrence was approved by the US. This pill which also goes by the market name Xpovio has been found to be. Drugs Approved for Bladder Cancer.
The Food and Drug Administration has approved a new cancer drug that is the first to be designed from the start to fight a specific genetic mutation not a traditional cancer type. Abraxane Paclitaxel Albumin-stabilized Nanoparticle Formulation Afatinib Dimaleate. New classes of drugs developed using new technologies provide new treatment options and hope to patients with fatal diseases.
Just imagine the difficulty of raising the tens of millions of dollars needed to get a new cancer drug into the approval pipeline when prospective investors see the FDA deny a drug with documented efficacy as was done recently with Provenge. It took the FDA more than a year to finally pull the breast cancer approval from Roches blockbuster drug Avastin. Approximately one-third of cancer drugs are approved based on response rate RR-the percentage of patients whose tumors shrink beyond an arbitrary threshold-typically assessed in a single-arm study.
Original NDA and Original BLA Approvals by Month. A retrospective review of FDA. FDA Approves First Drug for Cancers with a High Tumor Mutational Burden The US Food and Drug Administration FDA has expanded the approval of the immunotherapy drug pembrolizumab Keytruda to include any cancer with a high tumor mutational burden TMB-H.
Kloxxado naloxone hydrochloride nasal spray is an opioid antagonist. The drug is approved for patients who have received one or more prior treatments for their cancer the FDA announced. New skin cancer drug approved by FDA Odomzo is a pill for locally advanced basal cell carcinoma.
Refer to page 7 for the complete story of the FDAs denial of Provenge. And tentative approvals of ANDAs and NDAs. Drugs Approved for Anal Cancer.
Food and Drug Administration has granted approval to a blood cancer drug. All Approvals and Tentative Approvals by Month. Since the accelerated approval pathway was first enacted in 1992 drug withdrawals have been the exception rather than the rule.
Certain drugs are classified as new molecular entities NMEs for purposes of FDA review. Zynlonta loncastuximab tesirine-lpyl Injection. Extended adjuvant therapy is given after initial treatment to further reduce the risk that cancer will return.
Drugs approved by the FDA for specific types of cancer are listed on this page. See Drugs Approved for Childhood Cancers for a list specific to children. For example the cancer mortality rate in the US declined by 29 from.
Afinitor Everolimus Afinitor Disperz Everolimus Alecensa Alectinib Alectinib. Zynlonta loncastuximab tesirine-lpyl is a CD19-directed antibody and. Design setting and participants.
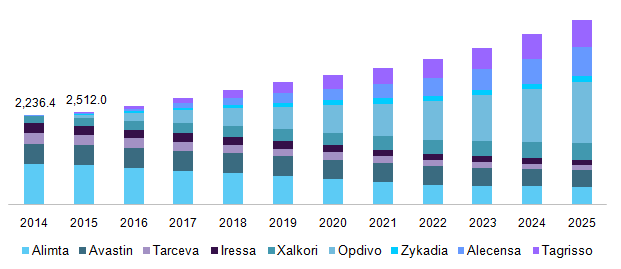
New Drug Approvals Kloxxado naloxone hydrochloride Nasal Spray. Drugs Approved for Non-Small Cell Lung Cancer. Alimta Pemetrexed Disodium Alunbrig Brigatinib Atezolizumab.
The pages are updated when new cancer drugs are approved. A Thursday statement from the FDAs oncology division noted how in the roughly 30-year history of the program just 6 of speedy approvals for cancer drugs have been pulled.