An FDA official Amy Abernethy formerly with real-world data venture Flatiron Health which was acquired by Roche noted that real-world evidence is used as a way to add to existing standards which falls in line with the TrialSite News understanding. The collaboration which was first established in 2014 builds on recent pioneering work by Pfizer and Flatiron Health to incorporate real-world evidence as part of a regulatory submission to expand the area of use for one of Pfizers breast cancer medicines.
 Flatiron Health Solving Cancer Through Data Analytics Digital Innovation And Transformation
Flatiron Health Solving Cancer Through Data Analytics Digital Innovation And Transformation
We posed the same 10 questions to FDA Pfizer and Flatiron Health.
Flatiron real world evidence. Established in 2012 Flatiron Health is focused on curation and development of real-world evidence. FDA recently created a framework for evaluating the use of real world evidence to support additional indications for already approved drugs as well as to satisfy drug post-marketing study requirements. The report published last.
We believe that the use of real-world evidence holds the potential to significantly accelerate cancer research and we are proud to expand our relationship with Pfizer Oncology a leader in this space In April 2018 Swiss drugmaker Roche completed its previously announced acquisition of Flatiron Health for 19bn 153bn. The new agreement builds upon a project started in 2016 to help the FDA better. Through clinical and data science we translate patient experiences into real-world evidence to improve treatment inform policy and advance research.
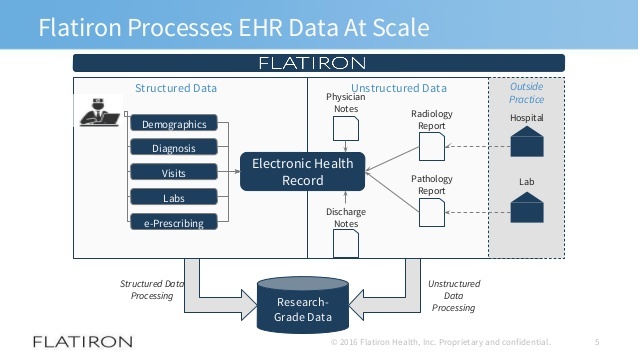
Flatiron Health is the leading source of oncology real-world data and methods dedicated to advancing knowledge of how evidence derived from EHRs can improve patient care and inform decisions. Flatiron Health acquired by Roche. Ibrance palbociclib was initially approved in 2015.
Additionally they have created an Epic App that allows for physicians to see evidence based therapy options and pulls. It is a kinase inhibitor now approved in combination with an. Deloitte has been conducting benchmarking surveys of the pharmaceutical industry since 2017 to track trends in strategic focus investments challenges and opportunities for delivering value with real-world data RWD and real-world evidence RWE.
The framework lays out the fundamentals of the agencys approach to developing guidances for using real world data in drug regulation. Here is what came back. FDA and Flatiron extend real world evidence collaboration Roche-owned specialists at forefront of big data analysis The FDA is pushing ahead with efforts to integrate real world evidence RWE into its regulatory decision-making and has an unveiled an extended collaboration with specialist Flatiron to help achieve this goal.
Flatiron Electronic Health Record Database study of EGFR TKIs Erlotinib was the most widely prescribed first-line therapy for EGFR Mut NSCLC in this study with similar efficacy observed for erlotinib- and afatinib-treated patients. Was this the first approval based at least in part on real world evidence in oncology. NEW YORK--BUSINESS WIRE-- Pfizer Inc.
Flatiron Health is a healthtech company dedicated to helping cancer centers thrive and deliver better care for patients today and tomorrow. Flatiron Health is working to transform oncology by using real world data generated from EHRs across the US that allows researchers to use synthetic control arms in their clinical trials. This study characterizes how two core real-world evidence RWE attributes the ability to accrue large patient cohorts and to aggregate longitudinal data throughout relatively long follow up periods can address the shortcomings found in clinical trial evidence and potentially lead to more precise evaluations of the impact of new technologies.
Together we can be smarter. NYSEPFE today announced the peer-reviewed publication of real-world evidence RWE demonstrating that first-line therapy with IBRANCE palbociclib in combination with letrozole was associated with improved real-world progression-free survival rwPFS and overall survival OS in women with hormone receptor. Flatiron team members discuss the evolving role of machine learning and natural language processing NLP in real-world evidence along with appropriate applications for these technologies.
February 27 2019 - The FDA and Flatiron have announced a two-year extension of their Information Exchange and Data Transformation INFORMED Program partnership to leverage real-world evidence RWE and clinical trial data to improve outcomes for cancer patients. Learn more about machine learning and AI. RWD and RWE have remained a C-suite strategic priority for pharmaceutical companies.