The study results page includes the. Approximately 42 of global participants and 30 of US.
 Clinical Trial Design And Dissemination Comprehensive Analysis Of Clinicaltrials Gov And Pubmed Data Since 2005 The Bmj
Clinical Trial Design And Dissemination Comprehensive Analysis Of Clinicaltrials Gov And Pubmed Data Since 2005 The Bmj
The ability to produce comparable clinical or other results including our stated rate of vaccine effectiveness and safety and tolerability profile observed to date in the remainder of the trial or in larger more diverse populations.
Clinical trial results. ClinicalTrialsgov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. None of the materials on this website may be reproduced modified distributed transmitted republished displayed or performed unless prior written approval is obtained by Clinical Trial Results. Publication of the results of clinical trials.
Breast Cancer Epidemiology Treatment and Prevention. De-identified participant data will be made available to bona fide researchers registered with an appropriate institution within three months of publication. On the advanced search page change the Study Results option from All Studies to Studies With Results.
A breakdown of the diversity of clinical trial. Competition to create a vaccine for COVID-19. The regulation of clinical trials aims to ensure that the rights safety and well-being of trial subjects are protected and the results of clinical trials are credible.
Monday November 16 2020 Promising Interim Results from Clinical Trial of NIH-Moderna COVID-19 Vaccine 3D print of a spike protein of SARS-CoV-2 the virus that causes COVID-19 in front of a 3D print of a SARS-CoV-2 virus particle. For example clinical trial results confirming the efficacy of Erbitux in the up to 65 of mCRC patients with the KRAS wild-type tumors indicating an intact EGFR pathway were presented in January at the American Society of Clinical Oncology Gastrointestinal Cancers Symposium ASCO GI. The information given below and the relevant links to the clinical data fulfil the requirements of Articles 71-73 of the Ordinance on Therapeutic Products Therapeutic Products Ordinance TPO and supplement the information published in association with the Swiss marketing authorisation for the.
If youre looking for a specific studys results you can narrow down the trials in the Other Terms box. Go to the Advanced Search page from their homepage. The EUA for the Moderna COVID19 Vaccine is in effect for the duration of the COVID19 EUA declaration justifying.
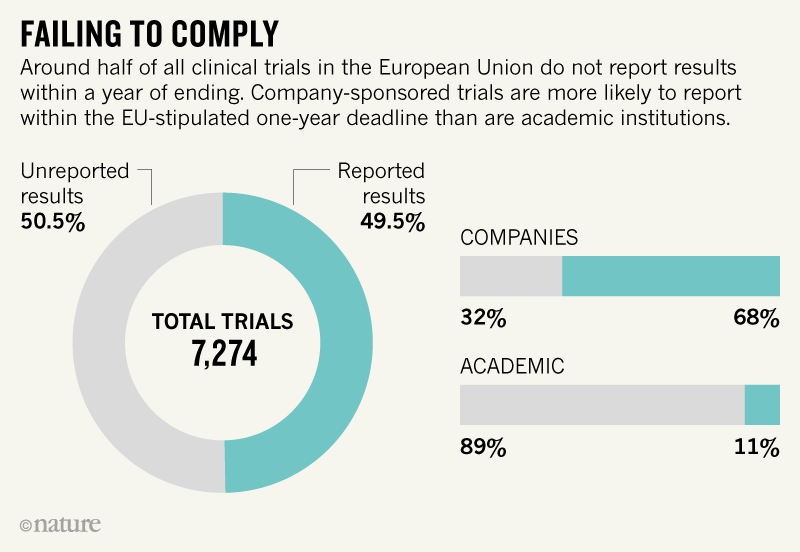
Explore 377550 research studies. The European Union Clinical Trials Register allows you to search for protocol and results information on. Posting of clinical trial summary results in European Clinical Trials Database EudraCT to become mandatory for sponsors as of 21 July 2014.
In 1976 Bonadonna and colleagues reported. Interventional clinical trials that are conducted in the European Union EU and the European Economic Area EEA. When the results of a study have been posted on ClinicalTrialsgov the Status column of the search results list includes the note Has Results.
Despite early roadblocks from clinical trial results with alginate hydrogel therapies the versatility of. Clinical trials conducted outside the EU EEA that are linked to European paediatric-medicine development. Participants have racially and ethnically diverse backgrounds and 41 of global and 45 of US.
These risks and uncertainties include but are not limited to. Clinical trials results Clinical trials allow researchers to determine whether a new way to prevent or treat a disease is safe and effective for human beings. Participants are 56-85 years of age.
As of 21 July 2014 it will become mandatory for sponsors to post clinical trial results in the European Clinical trials Database EudraCT managed by the European Medicines Agency EMA. Clinical Trial Results The Moderna COVID19 Vaccine has not been approved or licensed by the US Food and Drug Administration FDA but has. The spike protein foreground enables the virus to enter and infect human cells.
Clinical Trial Results Essential for patient safety. Clinical Trial Results Volume 1. This critical stage of creating a new medicine or vaccine follows many years sometimes decades of research.
However the steering committee will need to be satisfied that any proposed publication is of high quality honours the commitments made to the study. To view the study results click on the study title to view the record. The Phase 3 clinical trial of BNT162b2 began on July 27 and has enrolled 43661 participants to date 41135 of whom have received a second dose of the vaccine candidate as of November 13 2020.
You can also click on Has Results in the list of studies to go directly to the study results. As described in the protocol the trial steering committee will facilitate the use of the study data and approval will not be unreasonably withheld. Heres a step by step guide on how to find the result of a study on ClinicalTrialsgov.
Clinical trials are studies intended to discover or verify the effects of one or more investigational medicines. Then click on the Study Results tab of the study record to view the results. The ability to meet the pre-defined endpoints in clinical trials.
All such requests should be forwarded to Susan J.

