Moncef Slaoui PhD chief of the White House task force to develop a. Approving a vaccine in the US.
 Clinical Trials 101 Your Guide To The Gold Standard Of Research
Clinical Trials 101 Your Guide To The Gold Standard Of Research
More than 96 of participants received both.
Phase 4 vaccine trial. Objective Phase IV trials are often used to investigate drug safety after approval. Senate ratifies 2nd coronavirus pandemic relief package. September 4 Phase 1 trials of this vaccine were registered in June 2020 but no results have have been published in the scientific literature.
Usually takes years but COVID-19 vaccines are moving through in. The number of people participating in clinical studies grows along with our understanding of the investigational medicine and the research continues as long as the potential benefits outweigh the risks. These include one being developed by AstraZeneca in partnership with the.
A breakdown of the 4 COVID-19 vaccine trials in late-stage testing. According to MarketWatch there are only four major vaccine candidates right now that are currently in Phase III trials. The trial enrolled 30420 volunteers who were randomly assigned in a 11 ratio to receive either vaccine or placebo 15210 participants in each group.
Once Phase 1 trials were completed companies began producing vaccines while Phase 4 trials were ongoing so companies did not wait until trials were completed as is the usual norm. However little is known about the characteristics of contemporary phase IV clinical trials and whether these studies are of sufficient quality to advance medical knowledge in pharmacovigilance. Length of clinical trials.
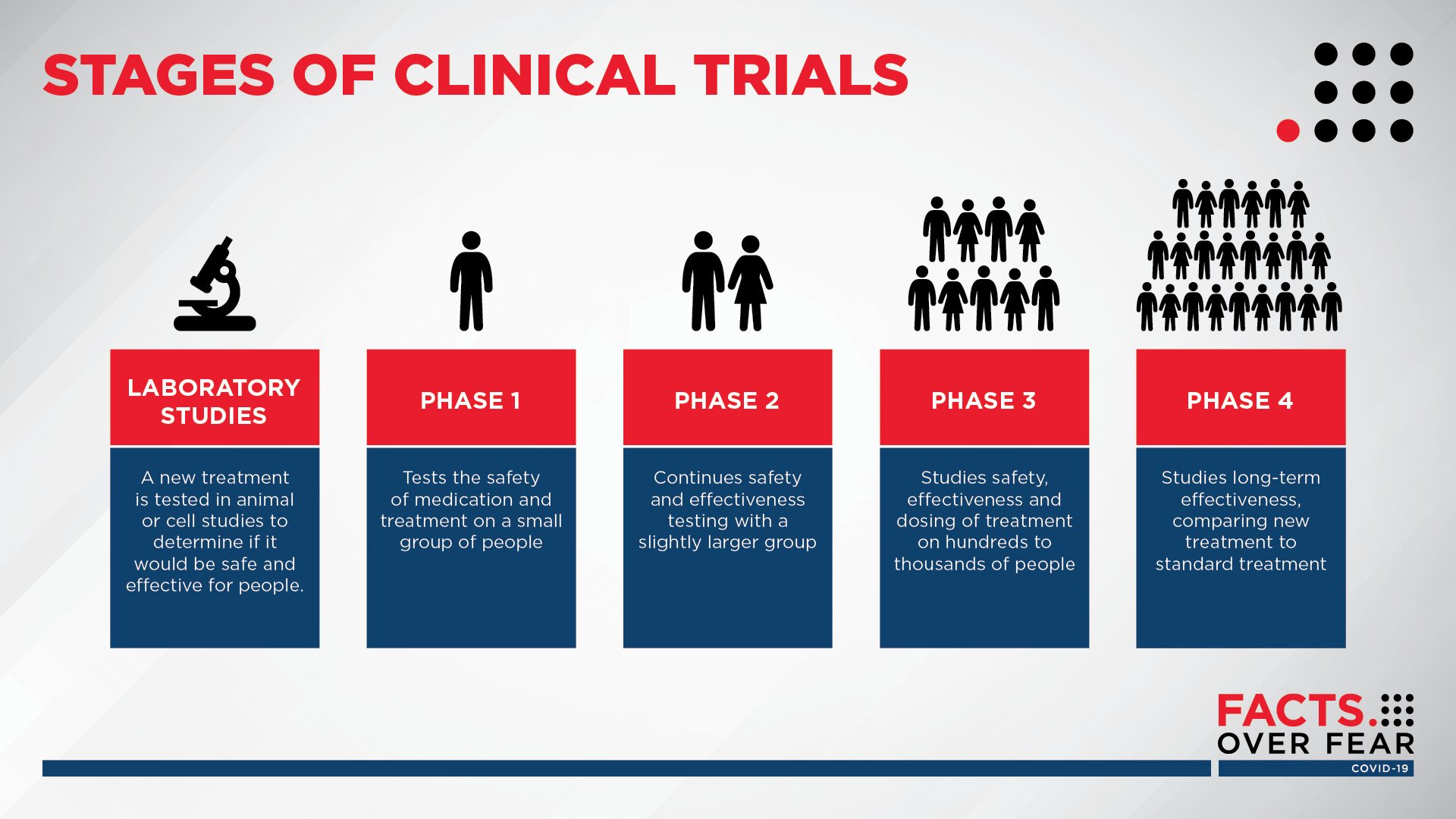
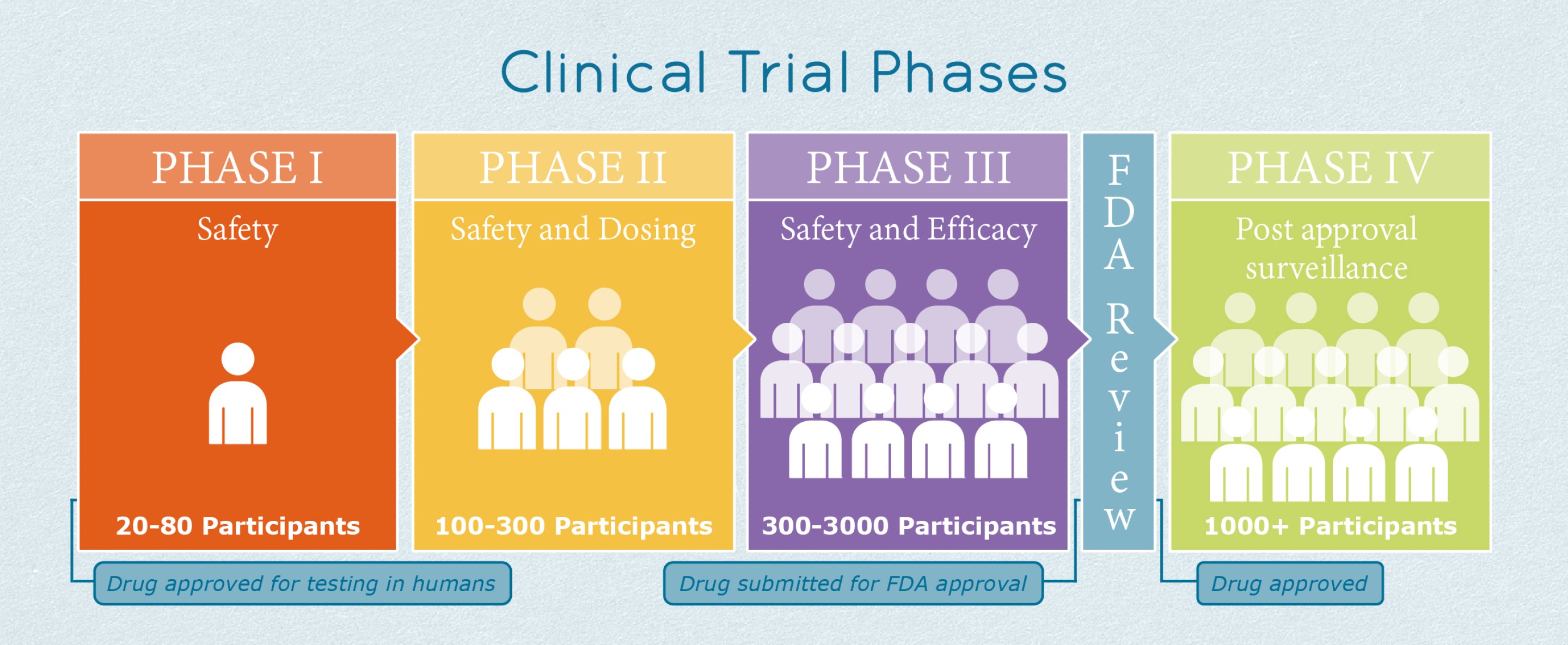
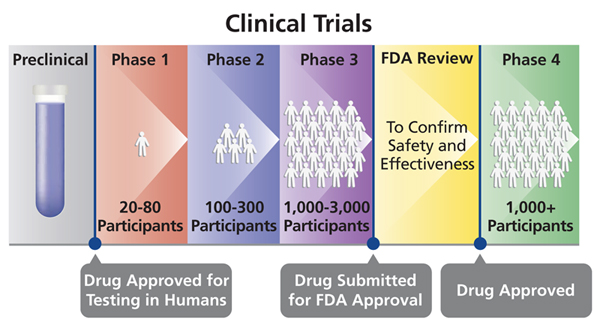
The process of learning about and developing an investigational medicine is divided into four phases. Despite this Russian President Vladimir Putin announced regulatory approval of the vaccine to be called Sputnik V in foreign markets. A fourth Phase 3 clinical trial evaluating an investigational vaccine for coronavirus disease 2019 COVID-19 has begun enrolling adult volunteers.
Pfizer Vaccine BNT162b2 Results of Phase 3 study of mRNA-based COVID-19 vaccine candidate BNT162b2 met all of the studys primary efficacy endpoints. Phase IV clinical trials happen after the FDA has approved medication. Thats right even after researchers have answered the big questions they keep studying the vaccine.
While it was doing away with the phase 4 trials the DOH said that as safeguards it would conduct safety and effectiveness surveillance and keep a database of recipients of the drugs and vaccines. What are the 4 phases of vaccine clinical trials. We aimed to determine the fundamental characteristics of phase IV clinical trials.
Investigators use this phase to. This phase involves thousands of participants and can last for many years. The trial is designed to evaluate if the investigational Janssen COVID-19 vaccine JNJ-78436725 can prevent symptomatic COVID-19 after a single dose regimen.
At first very few people receive the medicine being studied. Phase 4 This step happens after the FDA approves the vaccine. Once a vaccine is in widespread use data collection on its safety as well as how well it is working continues to be collected in what is known as phase 4.
Katie Adams - Thursday October 22nd 2020 Print Email. Mass vaccination in Russia is expected to start in October 2020. To evaluate the safety and immunogenicity of the three consecutive lots of an seasonal split influenza vaccine Anflu in adults a randomized double-blind and controlled clinical trial was conducted in 560 subjects in Tianjin City of China.
 Covid 19 Q A Sanford Research On Clinical Trials Vaccines Sanford Health News
Covid 19 Q A Sanford Research On Clinical Trials Vaccines Sanford Health News
 Covid 19 Vaccines Development Evaluation Approval And Monitoring European Medicines Agency
Covid 19 Vaccines Development Evaluation Approval And Monitoring European Medicines Agency
 Opinion How Long Will A Vaccine Really Take The New York Times
Opinion How Long Will A Vaccine Really Take The New York Times
 Phase Iii Ipf Clinical Trials Ild Collaborative
Phase Iii Ipf Clinical Trials Ild Collaborative
 Clinical Trials And Results Sanofi Uk Sanofi In The United Kingdom
Clinical Trials And Results Sanofi Uk Sanofi In The United Kingdom
 First In Human Covid 19 Vaccines Tales Of Phase 1 Clinical Trials Past Absolutely Maybe
First In Human Covid 19 Vaccines Tales Of Phase 1 Clinical Trials Past Absolutely Maybe
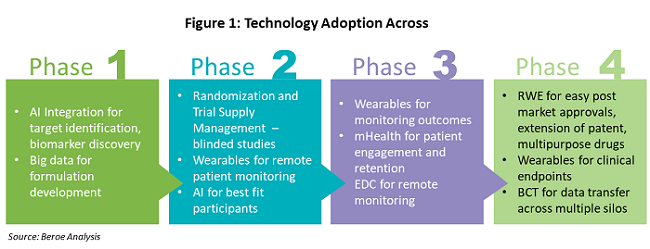
 Analyzing The Top Clinical Trial Technology Trends
Analyzing The Top Clinical Trial Technology Trends
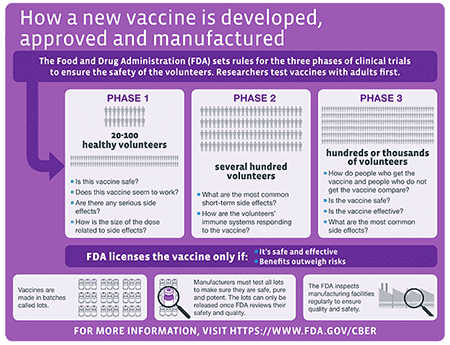 Ensuring The Safety Of Vaccines In The United States Cdc
Ensuring The Safety Of Vaccines In The United States Cdc
 What History Tells Us About Vaccine Timetables
What History Tells Us About Vaccine Timetables
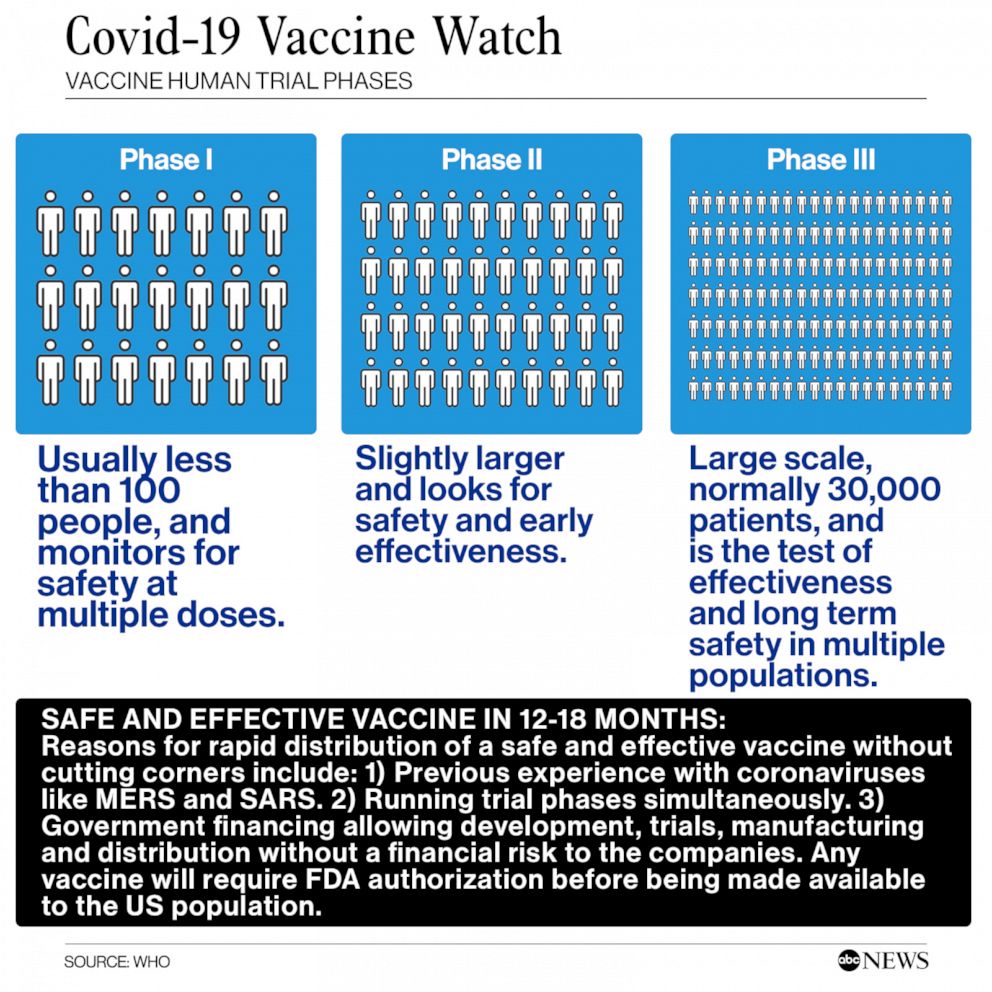 Human Trial For Coronavirus Vaccine Launched By Moderna Enters Phase 3 Abc News
Human Trial For Coronavirus Vaccine Launched By Moderna Enters Phase 3 Abc News
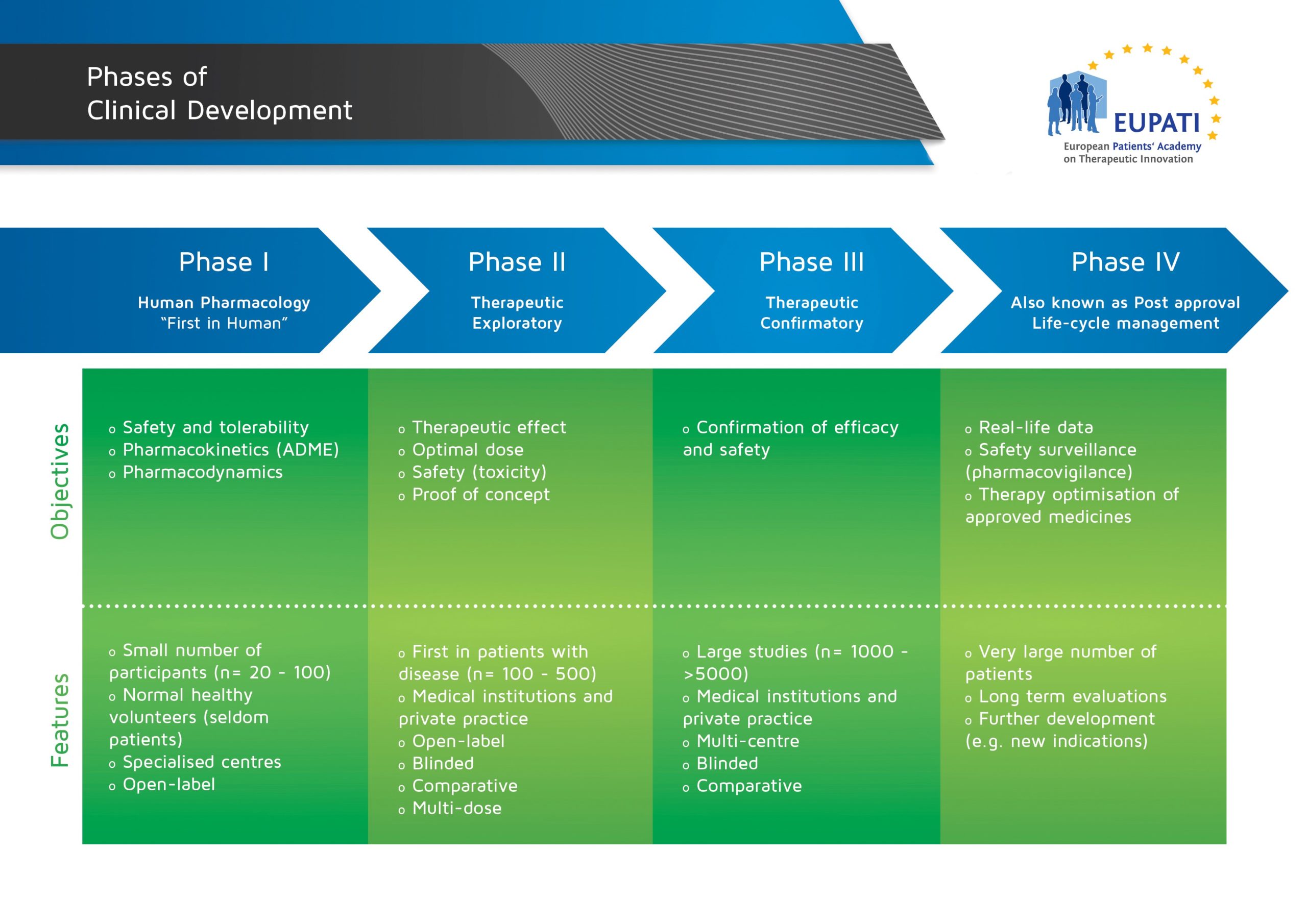 Basics Of Early Clinical Development Eupati Toolbox
Basics Of Early Clinical Development Eupati Toolbox
 How Do Clinical Trials Progress Cancer Institute Nsw
How Do Clinical Trials Progress Cancer Institute Nsw


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.